|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
4 Releases and Commercialization4.1 Safety AspectsIn the early 1970s, the introduction of molecular gene transfer technologies in bacteria gave rise to questions among scientists about possible risks of this new technology. Following the first successful gene transfer in 1975, the scientific community discussed these questions on the conference of Asilomar and decided to set up rules for the self-control of laboratory experiments in order to avoid any inadvertent generation of harmful effects. This resulted in a number of national and international recommendations, guidelines and legislations, e.g. the OECD report Recombinant DNA Safety Considerations (1986). With the accumulation of experience with transgenic organisms and the better understanding of genetics and gene transfer, among scientists the opinion began to prevail that genetic engineering itself does not pose specific novel risks and that regulatory review should focus on the hazard potential related to characteristics of the transformed organism and the introduced traits, not on the process by which it has been created. No accidents or other deleterious effects of transgenic plants or other transgenic organisms have been reported so far. However, in the public there is still concern about the risks of genetic engineering, sometimes leading to complete refusal of any application of this technique. In the following, general safety aspects related to the release of transgenic crops and specific safety aspects of the methods used to achieve disease or pest resistance are reviewed. 4.1.1 Gene Transfer4.1.1.1 Outcrossing to other PlantsGenes may be transferred by pollen to other cultivars of the same crop or, more rarely, to wild or weedy relatives that grow in the agricultural or natural environment. The chances for movement of genes from a crop to a wild relative depend on a number of coincidences. These events are as follows.
If one of these events does not occur, outcrossing is not an event of concern. Gene transfer by pollen to a wild relative within a population can be considered as analogous to a mutation arising in the population. Whether the transferred trait becomes initially established in the population depends more on chance effects than on fitness, since the great majority of mutants are lost from the population because of genetic drift, even if the gene confers specific advantages (Gale 1990, cited in [38]. Only repeated pollination will lead to the integration of the trait into the wild genetic background. Whether the new trait will spread in the population or get lost again depends on the effect the transferred gene has on the wild relative. If the effect confers a selective advantage on the hybrid plant under the prevailing environmental conditions, the hybrid plants may spread and could cause changes in the species composition. Selective advantages might be caused by greater resistance to a disease or an insect pest, or greater tolerance to environmental stresses present in the agricultural or natural environment. [38] A weed may be defined as "a plant which is not intentionally sown, whose undesirable qualities outweigh its good points" (Granatstein 1989, cited in [39], although several other definitions exist. A wild relative that becomes a weed because of the introduced trait, or a weed with an increased weediness due to the introduction of a transgene by outcrossing with a transgenic crop may be identified as a safety concern. Therefore, measures must be taken to prevent outcrossing of genes which could increase the fitness of wild relatives, if it is possible that all the above mentioned conditions are met simultaneously. These measures may include isolation of test sites from areas inhabited by relatives, control of pollen sources by mowing or herbicides, using sterile plant lines unable to produce seeds in the case of clonally propagated plant lines, and others. [38] The recently developed method of plastid transformation (see chapter 3.1.1) offers advantages for the containment of transgenes. In plastid transformation, the transgene is introduced into the genome of the plant chloroplasts. As chloroplasts are inherited maternally only in almost all crop species, pollen produced by plants carrying the transgene in the plastid genome do not contain the transgene. [11] 4.1.1.2 Gene Transfer to MicroorganismsAs discussed above, Agrobacterium tumefaciens is a widely used bacterial vector in plant genetic engineering. Some Agrobacteria carrying the transgene may be present in plant cells after transformation and could exit through the roots into the soil, where the transgene could be passed on via conjugation to other bacteria or to other plants via infection. Therefore, the absence of Agrobacteria is an important requirement for the release of transgenic plants into the environment. Another possible mechanism for gene transfer is the uptake of DNA released by decaying plants through microorganisms. However, for this "horizontal gene transfer" event to occur, several obstacles would first have to be overcome. Successful transfer of a functional gene must be considered a very rare
event. As in the case of outcrossing to related plants, the transferred
gene will only be maintained in the bacterial population if it confers a
selective advantage, which makes successful horizontal gene transfer even
more unlikely. However, evolutionary considerations indicate that gene
transfer from plants to bacteria may have taken place, as some bacterial
enzymes are known to have a sequence more similar to eucaryote sequences
than to procaryotic types. Another possible type of horizontal gene
transfer, viral recombination with transgenic plant mRNA transcripts,
is discussed in chapter 4.1.4. [40] BATS-Report 3/94
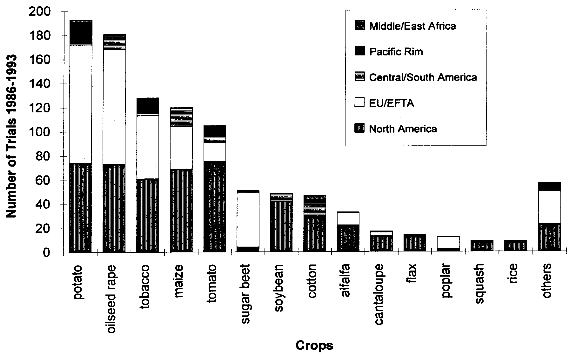
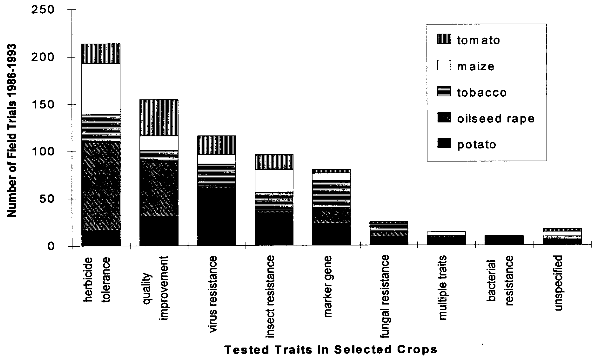
( 4.1.2 Increased WeedinessA crop is considered a weed when it carries over (grows in subsequent seasons) or establishes in neighboring fields and competes with subsequent crops. Most crop species depend completely on human nurturing and are therefore unable to compete successfully with plants adapted to the natural environment. There are, however, examples of crops that are minor weeds in natural or agricultural ecosystems, examples being oilseed rape, sunflower and rye. According to Baker (1974, cited in [38]), weediness is a multicharacter attribute and the addition of one gene is unlikely to cause a crop to become a weed. In contrast, Fitter et al. (1990) and Williamson et al. (1990, cited in [38]) suggest that the alteration of one gene may indeed be enough to change a crop into a weed. If a crop species has very few weedy characteristics, the addition of one or a few genes would be unlikely to cause that crop to become a weed problem. Special attention might be warranted where the crop has weedy characteristics or the added genes might be expected to improve the crops competitive ability in natural or agricultural ecosystems. [38] An indication of the tendency of certain cultivars to carry over may be obtained from previous experience (familiarity) with the crop, the introduced trait, the environment in which the plant will be grown, and their interactions. Standard agricultural practices like tillage, mowing, herbicide use and crop rotation are widely available for the control of weeds within the agricultural environment, including the control of carry-over of transgenic plant lines at the test site.[38] 4.1.3 Undesirable Phenotypic TraitsPresently, introduced genes can only be targeted to a predicted site by using the recently developed method of plastid transformation. Therefore, in most experiments the transgene integrates randomly into the plant genome. The introduction of foreign genes effects the plant metabolism directly through the new gene product, as well as through possible secondary interactions. Direct effects of the transgene products will be described in the chapters dealing with the different methods designed to achieve pest resistance. Here, only possible secondary effects of the transgene integration are discussed. Such secondary effects may arise from the interruption of plant genes resulting from the integration of the transgene, or changes in the expression rate of other genes caused by the introduction of new promoters and position effects. However, in this respect, transgenic plants are not basically different from products of traditional plant breeding, where secondary effects are known as well. One example is brown sorghum bred for bird-resistance, where a higher tannin content caused anti-nutritional properties of the seeds. Another example is a potato variety bred for resistance to the Colorado potato beetle which contained to much solanin, and was therefore not registered by the German Federal Cultivar Office. [40] As no predictions of secondary effects of new plant varieties (whether obtained by traditional breeding or with the help of genetic engineering) are possible, investigations have to be done after hybridization or gene transfer. Morphology, growth, yield, nutritional composition, and levels of potential toxins are assessed during development of a new variety, where only plants without undesirable phenotipical traits will be selected for further breeding. [40] 4.1.4 Specific Safety Aspects4.1.4.1 Strategies against InsectsBacillus Thuringiensis Toxin Bacillus thuringiensis (Bt) has been used as a biological pesticide for more than 50 years now, apparently without any deleterious effects on non- target insects, other animals and humans. [8] The spectrum of activity of an individual δ-endotoxin tends to be quite narrow, with a given δ-endotoxin being active only against a few (known) insects. Solubilization in the insect midgut, activation of the protoxin and toxin binding to the receptor in the midgut are steps which play a role in the specificity of a δ-endotoxin. [8] Possible toxic effects of Bt toxin expressed in transgenic plants have also been intensively investigated. No effects on humans or other mammals were found. For instance, rats were fed with the Bt toxin CrylA(b) corresponding to a human daily consumption of 2000 kg transgenic Bt-expressing tomatoes. No damage could be observed. Also a 90-day feeding trial with rats did not reveal any signs of adverse effects. [42] Several major pest species have shown their ability to adapt to Bt toxins, either in laboratory tests or in the field. Changes in the specificity of the toxin receptor seem to represent the major cause of resistance development. Insects are well known for their ability to develop resistance against insecticides and the development of resistance to Bt toxins is not specific for the expression of Bt toxin in transgenic plants. Different strategies are discussed for the management of insect resistance, when deploying Bt toxins through transgenic plants, e.g. developing and maintaining refuges for the survival of susceptible insects, growing mixtures of cultivars expressing different toxins, sequentially planting such different cultivars, expressing mixtures of toxins in one transgenic plant line, etc.. None of these strategies have proven their general suitability, and extensive field trials are necessary to further evaluate their advantages and disadvantages. [43] Protease Inhibitors, Amylase Inhibitors, and Lectins Protease and amylase inhibitors apparently act by interfering with the insect digestive process. The conditions under which digestive processes take place differ considerably between insects and mammals and between different orders of insects, e.g. regarding midgut pH. Therefore, protease inhibitors affecting insect enzymes would not necessarily also effect enzymes of human consumers. Cowpea trypsin inhibitor (CpTI) e.g. apparently lacks mammalian toxicity.[15] However, these inhibitors may also become harmful to beneficial insects if they are constitutively expressed at the levels necessary to accomplish protection, and their potential impact must therefore be assessed carefully before large-scale field trials take place. [9] Protease inhibitors, amylase inhibitors and lectins are examples of proteins which are part of the natural plant defense system. They are therefore already present in our daily diet. However, most (genetically unaltered) plants contain substances which can be toxic to humans or other consumers or which cause allergies to susceptible people. For instance, lectins are known to be toxic to both mammals and birds when ingested without heating prior to consumption.[16] Therefore, it could still be possible that such insecticidal substances cause harm to consumers, especially if they are produced in higher amounts than usual in transgenic plants. As the products of the transgene are known, the toxic and allergenic effects can be evaluated prior to a potential commercialization. This makes sure that transgenic crops reaching the marketplace are as safe as other food sources. 4.1.4.2 Strategies against VirusesViral Coat Proteins In coat protein-mediated protection (CPMP), resistance to viruses is achieved by expression of a viral coat protein in transgenic plants. A potential adverse effect of CPMP comes from the possibility of heterologous encapsidation (also called transcapsidation or heteroencapsidation) resulting from the following mechanism: When a transgenic plant synthesizing a viral coat protein is infected with another virus, the replicated nucleic acid of the second virus may be packed (encapsidated) into the viral coat produced by the plant. Because encapsidation is a relatively specific phenomenon, heterologous encapsidation is unlikely except for closely related viruses or strains of viruses. As the coat protein plays an essential role in virus transmission by vectors such as aphids or nematodes, the proposed mechanism could affect the transmission properties of the infecting virus Indeed, this effect could be shown in the laboratory with transgenic tobacco plants expressing the coat protein of an aphid transmissible plum pox potyvirus (PPV) strain. After infection with an aphid- nontransmissible zucchini yellow mosaic virus (ZYMV) strain, aphid- transmissible PPV coat containing ZYMV genome was found, and the combination was found to be aphid-transmissible. [23] A situation possibly leading to heteroencapsidation also exists in nature, when a plant cell is infected by two viruses simultaneously. In this case, the genome of one virus could be packed into the coat protein of the second. However, it is important to note that heterologous encapsidation does not lead to a change in the viral genome and therefore is a "one generation problem". New viral transmission properties - if any - would be lost again after one infection cycle and not inherited to subsequent generations. [23] Another potential environmental risk could arise from the recombination of viral RNA with plant messenger RNA (mRNA) from genetically engineered viral coat protein genes. Viral recombination could lead to viruses with new traits, e.g. altered virulence. A mechanism for viral recombination has been termed copy choice or template switching and is thought to be a quite general phenomenon, although not involved in the normal replication circle. In nature, there is evidence for incorporation of sequences of plant or animal RNA in plant and animal viruses. For example, the genome of the S-strain of potato leaf roll virus contains a 119-nucleotide segment of a plant chloroplast mRNA. There is at this time only one case described of plant viruses in which natural incorporation of host sequences may have led to changes in virus pathogenicity. [23] Viral recombination in transgenic plants could be shown in a recent laboratory experiment. [44] Whether large-scale cultivation of transgenic plants could increase the frequency of viral recombination or heterologous encapsidation is not known. [23] Other Virus-Derived Genes As the other approaches to achieve virus resistance do not lead to production of a coat protein, no heteroencapsidation is possible. Several examples have been described where high levels of resistance with low expression rates of defective viral RNAs or antisense RNA without production of functional proteins has resulted in high levels of resistance. These strategies are unlikely to cause risks higher than in non-transgenic plants. Satellite RNA Certain satellite RNAs of cauliflower mosaic virus induce a lethal necrosis in tomato and certain related species. Outbreaks of lethal necrosis are usually limited, but have reached epidemic proportions in Italy in 1988 and 1989. Sequence differences between satellite RNAs that reduce disease symptoms and those that cause lethal necrosis can be very small. In some cases, a single point mutation caused a nonnecrogenic satellite RNA to become necrogenic in tomato. [23] Therefore, the satellite RNA strategy is thought to be associated with risks and needs further investigation. [9] 4.1.4.3 Strategies against Fungi and BacteriaThe genes used for resistance against fungi mostly come from plants and their products are therefore not new in our diet. This does not necessarily mean that they cannot cause problems, as already discussed in chapter 4.1.3. Careful assessment of toxicity and allergenic potential prior to large-scale cultivation and use as human or animal food can ensure that transgenic crops are as safe as conventional crops. Basically the same is true for genes which are derived from organisms not used as food, e.g. cecropin genes from insects against bacteria. However, the products of genes which are not normally present in food may need an even more careful evaluation, because less experience with these compounds exists. 4.1.4.4 Transgenic BaculovirusesBaculoviruses have been genetically altered to decrease the time they need to kill plant-feeding insects. Effects on non-target organisms like other insects or humans and spread of transgenic baculoviruses have been in the center of interest. Baculoviruses were found to be non-pathogenic to mammals, other vertebrates, and plants, and are unable to penetrate through nuclei of mammalian cells. Most baculoviruses are active only on a single insect family or genus. The restricted host range strongly reduces the risks of deleterious effects on beneficial and non-target organisms. For field releases, transgenic viruses with a selective disadvantage relative to the wild-type can be used, so that recombinant strains are rapidly competed out by wild-type viruses, if application of transgenic viruses is discontinued. The shorter time to kill of transgenic viruses is also a selective disadvantage, because less new viral particles are produced before the host insect dies. [33] However, repeated commercial applications of transgenic baculoviruses at a single site may lead to high levels of viruses in the soil, and the ecological consequences of this are currently unclear. [45] 4.1.4.5 Transgenic BacteriaInactivated Bacteria Expressing Bt Toxin The genes for the δ-endotoxin of Bacillus thuringiensis (Bt toxin) have been transformed into Pseudomonas fluorescens to avoid the rapid degradation of Bt toxin which occurs when Bacillus thuringiensis is used as an insecticidal spray. Since the transgenic P. fluorescens are killed before spraying and no living transgenic microorganisms are released into the environment, this approach has the advantage of relative freedom from environmental and safety concerns associated with field releases of living transgenic organisms. In 1985, the U. S. Environmental Protection Agency (EPA) approved testing of the inactivated P. fluorescens with the first field release permit for a transgenic product. [36] Bacterial Endophytes Expressing Bt Toxin In order to increase the effect of Bt toxins, the corresponding genes have also been introduced into bacterial endophytes (bacteria that live inside of plants). As these endophytes produce the Bt toxin inside the plant, the toxin is also effective against insect pests feeding inside the plant, and it is not quickly degraded like Bt toxin sprayed on plants. Clavibacter xyli spp. cynodontis (Cxc) has been used for Bt toxin delivery in the most advanced approach. Most of the available information about the biology of Cxc has been generated since 1986 in extensive laboratory, greenhouse, and field studies performed by the company Crop Genetics International (CGI) and cooperators for submission to the U.S. Environmental Protection Agency (EPA) and the Animal and Plant Health Inspection Service (APHIS) in support of development and registration of products for maize and rice protection. [37] These studies show that in nature a living host is required for replication of Cxc, that Cxc is short lived in maize plant debris and cannot be detected in soil or irrigation runoff water around inoculated plants. Volunteer maize seedlings originating from inoculated plants did not become colonized. Xylem-feeding leafhoppers and flea beetles, both of which are known vectors of bacterial pathogens of the plant vascular system, and several other tested insect species, were found to be unable to transmit Cxc to uninfested plants. The results of these studies have provided evidence that soil, water, colonized plants, plant debris and insects are unlikely to serve as a source of persistent Cxc inoculum for weeds, neighboring fields or subsequent crops in the same field. Apparently, Cxc is transmitted in nature by vegetative propagation of bermudagrass via runners, and only through mechanical plant injury (animal grazing, mowing) does it occasionally move to other plants. [37] The engineered genes are spontaneously lost from Cxc at low frequency due to homologous recombination with sequences originally present on the Cxc chromosome. The resulting segregants (cells which have lost the introduced trait) appear to be identical to the wild-type Cxc strain. Segregants grow more quickly than recombinants, so that selection eventually leads to complete loss of bacteria containing the recombinant genes. The progress of this reversion phenomenon is sufficiently low to ensure product performance within a growing season, but occurs rapidly enough to insure that Bt toxin genes would not persist in the Cxc population. [37] 4.2 Regulatory Framework4.2.1 Case Study USAU.S. federal policy does not view genetically engineered organisms as fundamentally different from those traditionally isolated from nature and introduced into new environments, or varieties produced through programs of breeding and selection. Not the technique by which certain traits have been engineered into an organism is in the focus of the regulation, but the exclusion of hazards resulting from the engineered organism. Consequently, the release and commercialization of transgenic organisms is regulated under existing laws, which have been partially adapted to the new techniques. The three main administrations concerned with transgenic organisms are the U.S. Department of Agriculture (USDA), the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA). In short, the USDA deals with plant pest traits which may be exhibited by the genetically altered organism, the EPA investigates primary and secondary effects of potentially toxic compounds (especially pesticides) expressed by transgenic organisms, and finally, the FDA analyses other compounds not covered by the EPA. The three departments work together in order to minimize duplications of efforts in areas of overlap such as the assessment of environmental data and the preparation of environmental documentation. In the following, the different focuses and aims of these organizations are described in more detail. 4.2.1.1 U.S. Department of AgricultureThe U.S. Department of Agriculture (USDA) has a statutory mandate to protect U.S. agriculture against the introduction and dissemination of plant pests. Under the Federal Plant Pest Act (FPPA) and the Plant Quarantine Act (PQA), the Animal and Plant Health Inspection Service (APHIS), which is the regulatory arm of the USDA, regulates the movement into and through the United States of plants, plant products, plant pests, and any product or article that may contain a plant pest at the time of movement. In the FPPA, a plant pest is defined as "any living stage of any insects, mites, nematodes, slugs, snails, protozoa, or other invertebrate animals, bacteria, fungi, or parasitic plants or reproductive parts thereof, viruses, or any organisms similar to or allied with any of the foregoing, or any infectious substances, which can directly or indirectly injure or cause disease or damage in any plants or parts thereof, or any processed, manufactured, or other products of plants". Genetically engineered organisms are deemed "regulated articles" under the regulations if the gene donor organism, the recipient organism, or the vector organism meets the definition of plant pest. The focus of the review under the regulations is to certify that the there is no plant pest risk from shipping or field testing the organisms even though the organism was developed through the use of genetic material from a plant pest. As many plants are transformed using the "disarmed" plant pest Agrobacterium as a vector, and/or use genes derived from pathogens (like viral coat proteins), virtually all transgenic plants fall under the APHIS regulations. To apply for a field test permit for a genetically engineered organism under the regulations, an applicant must complete an APHIS form and supply the information requested in the form. A permit application must contain sufficient information on the crop plant, the nature of the genetic modification, and the protocol for conducting the field trial to allow for an evaluation of any potential plant pest risk or environmental effects that may result from the field trial. To assist applicants in preparing an application, a user's guide has been developed that provides a detailed description of the elements to consider in writing an application. [46] The application should be submitted at least 120 days in advance of the proposed field test. Within 30 days after the application is received by APHIS, a copy of a preliminary assessment is sent to the state in which the test will take place, and the state agencies have 30 days after receiving the application to comment. A key component of the permit review process is the environmental assessment (EA), which contains a thorough accounting of the agency's analysis leading to a decision on whether to issue a permit. If the environmental assessment results in a finding of no plant pest risk and of no significant impact on the environment, the permit is issued. The EA is a public document that provides assurance that APHIS has fully considered the possible consequences of releasing the regulated article into the environment. EAs are available via Internet (see [16] for further information). Unlike the EPA, APHIS has no licensing authority to regulate the commercial use of genetically engineered products, but the "delisting" of the product as a plant pest is an important prerequisite for commercialization. [47] 4.2.1.2 Environmental Protection AgencyThree of the statutes administered by the U. S. Environmental Protection Agency (EPA) address the environmental and human health issues associated with the use of pesticides and other products that enhance agricultural production: The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), the Federal Food, Drug and Cosmetic Act (FFDCA), and the Toxic Substances Control Act (TSCA). Under FIFRA, the EPA has the authority to regulate development, sale, use, and disposal of pesticides. Pest control substances produced by genetically engineered plants or microorganisms are pesticides within the meaning of FIFRA and are therefore subject to FIFRA. To date, most transgenic organisms regulated under FIFRA express Bacillus thuringiensis δ-endotoxins. For a pesticide to be registered, FIFRA requires that the pesticide will not, when used in accordance with commonly recognized practice, cause "unreasonable adverse effects", taking into account 1he economic, social, and environmental costs and benefits of the use of [the] pesticide". Under the Federal Food, Drug, and Cosmetics Act (FFDCA), EPA is responsible for determining the amount of pesticide that may be present in raw or processed agricultural commodities when they enter commerce. The statute gives broad authority to protect against human dietary risks that might be posed by the use of pesticides (including pesticides produced by transgenic organisms) in food for humans or as feed for animals. FFDCA contains a concept that some substances in food may be "generally recognized as safe" (GRAS). A tolerance (the maximum level of pesticide residue allowable in a food or feed) is not required if a substance/pesticide is GRAS. GRAS status is based either on a safe record of use in food or evidence of safety and widespread agreement in the appropriate scientific community. The Toxic Substances Control Act (TSCA) authorizes EPA to acquire information on chemical substances and mixtures of chemical substances in order to identify and regulate potential hazards and exposures. EPA is required to screen new chemical substances prior to their introduction into commerce to identify unreasonable risks to human health or the environment. If EPA takes no action, the submitter may proceed to commercialization, and the substance may be listed in the inventory. Substances listed in the inventory are not new. Organisms, especially microorganisms, are viewed as mixtures of chemical substances in the TSCA and may therefore be subject to TSCA. EPA interprets a "new, microorganism to be one formed by deliberate combination of genetic material from source organisms classified in different taxonomic genera, also called "intergeneric microorganisms". An example of transgenic organisms that come under the authority of TSCA are nitrogen-fixing bacteria of the genus Rhizobium. [48] 4.2.1.3 Food and Drug AdministrationThe Food and Drug Administration (FDA) issued a policy statement on foods derived from new plant varieties, including genetically engineered plants, in 1992. This document stated that most substances added to food as a result of genetic modification are substantially similar to substances commonly found in food and therefore should not be subject to premarket "food additive" regulation under FDCA unless "objective characteristics raise questions of safety sufficient to warrant formal premarket review and approval." The implication of the document is that most substances added to food as a result of genetic modification would be considered as "generally recognized as safe" (GRAS) and would not require regulatory approval as a food additive. The FDA food policy establishes that substances in plants that properly meet the definition of pesticide under FIFRA will be addressed by the EPA. [47] 4.2.2 Case Study European Union/GermanyThe situation in the European Union (EU) is more complex than in the USA, as the EU consists of a number of independent states which have agreed to form a single market. They have conceded some of their powers to the EU, but essentially still operate as independent Member States. The Council of Ministers, which is the final decision-making body of the EU, has issued several directives concerning genetic engineering. Directives are binding as to the results to be achieved, but the form and the methods used to achieve the results are left to the national authorities who translate them into national law. [49] 4.2.2.1 European Union Directive 901220/ECThe main directive concerning the release of genetically engineered plants is the "Council Directive on the Deliberate Release into the Environment of Genetically Modified Organisms", known as "90/220/EC". The directive was adopted in 1990 and covers all genetically engineered microorganisms, animals and plants through all stages of release. All deliberate releases must be reviewed on a step-by-step, case-by-case basis and must be accompanied by an environmental impact assessment. Releases can only take place where a national approval procedure for experimental release already exists. There are considerable differences in how the member states have incorporated directive 90/220/EC into national law, and how this laws are interpreted. In many countries, the regulatory activity has been based on the premise that genetic engineering is a technique in its own and that there are unique products and of biotechnology which require special considerations. Consequently, some countries, e.g. Germany, have established a special genetic engineering act. This is in contrast to the situation in the USA described above, where not the fact that an organism has been genetically engineered, but the (novel) traits of the modified organism are in the focus of regulation. [49] As an example of a country with a genetic engineering act, the situation in Germany will be described in more detail. 4.2.2.2 Releases under the German Genetic Engineering LawThe German genetic engineering law (GenTG, Gesetz- zur Regelung von Fragen der Gentechnik) forms a binding framework for genetic engineering activities in Germany. Paragraphs 14-16 and 18 describe the procedures which must be followed for deliberate releases of transgenic organisms. The ZKBS (Zentrale Kommission for biologische Sicherheit, central biosafety committee) scrutinizes and evaluates the applications for releases. The ZKBS asks for the comments of the BBA (Biologische Bundesanstalt, federal biological institute), the Umweltbundesamt (federal environmental agency) and the agencies of the Bundeslaender (federal states) in which the field release will take place. If all legal conditions are met (e.g. all information required submitted), the application should be answered within three month . [49] The documents required for the application include the following data: [50] The data requirements for a field release in Germany are not fundamentally different from those in the USA, but the American approach seems to be more pragmatic and straightforward. In the USA, the procedures needed to obtain a field release permit are a well-established routine, while in Germany only a few permits have been issued so far. [50] 4.3 Field Trials with Transgenic PlantsField trials with transgenic plants world-wide are registered by the Green Industry Biotechnology Platform (GIBiP), an industry association of European companies which actively use biotechnology for the improvement of plant varieties. The advantage of the GIBiP database is that it applies the same definition for each entry, thus allowing an accurate comparison of the field trials in the 32 countries where field trials have been registered. One field trial is defined in the GIBiP database as "a single basic genetic strategy introduced into a single crop and tested in a single country by one company or institution in one year". Patricia Ahl Goy and John H. Duesing have analysed the information in the database and draw the following conclusions from the review of known activities world-wide from 1986 to 1993. [20] Up to the end of 1993, 1025 field trials with genetically manipulated plants (GMPs) have been conducted world-wide. From 1986 to 1991 in North America and the European Union/European Free Trade Association (EU/EFTA) the number of field trials was approximately the same. However, since 1992, North America has become leading area and accounts for 517 trials, slightly more than half of the world�wide total. As - according to the definition of "field trial" mentioned above - the field trials in different states of the U. S. are counted as one trial, but those in two different EU/EFTA countries as two, the number of field trials in the USA is underestimated compared to the EU/EFTA. Additionally, in North America more trials with multiple locations take place, and therefore the lead of North America is even larger. Ahl Goy and Duesing estimate that in 1993 single trials on over 1300 sites took place in North America, as compared to less than 200 in the EU/EFTA. Activity in Central and South America (6 % of all trials) has increased since 1991, principally serving as a counter season location to evaluate material also being tested in the Northern Hemisphere. The reasons why the EU/EFTA has fallen behind North America are unclear. According to Ahl Goy and Duesing [20] they could include differences in agricultural crops (many crops important in America like soybean, cotton, maize, flax and tomato are transformable since several years, while important crops in Europe like wheat, barley and sugar beet are more difficult to transform), the lower involvement of the European public sector in research with transgenic plants (see below), and in differences in the procedures for getting authorizations or approvals. 38 different transgenic plant species have been field tested. The model plant tobacco has been overtaken by crops of greater economic value. The five crops potato, oilseed rape, tobacco, maize and tomato total more than 100 trials each and constitute together 70 % of all trials. The other species tested up to the end of 1993 are sugar beet, soybean, cotton, alfalfa, cantaloupe, flax, poplar, squash, rice, birch, chicory, lettuce, petunia, cucumber, apple, cauliflower, sunflower, walnut rot, chrysanthemum, gerbera, sugarcane, asparagus, brassica sp., cabbage, eggplant, eucalyptus, kiwi, papaya, peanut, plum tree, spruce and strawberry. Given their economic importance, monocots such as rice and cereals are clearly underrepresented among trials to date because routine transformation of monocots lagged behind dicots. Genetically modified rice and maize were field tested only in 1990. The proportion of monocots is thought to rise during the next years. About one third of all field trials with the five most often test plants evaluated pest and disease resistance. Due to the early success of coat protein mediated protection and Bacillus thuringiensis toxins, virus resistance (16 % of trials with the five most frequently used crops) and insect resistance (13 %) are the most often tested traits in this sector. Despite the economic importance of fungal and bacterial diseases, the number of field trials with fungal and bacterial resistance is still low with 3 % and 1 %, respectively. The most commonly evaluated trait in plants still is herbicide tolerance (34 % of all field trials), with a decreasing trend relative to other traits. Besides the fact that genes conferring tolerance were among the first to be identified and transferred, the main reasons for this dominance are (1) a number of companies wish to expand the potential market for their herbicides, (2) herbicide tolerance serves as a selectable marker for the in vitro selection of other traits and for recovering the transgenic segregants in the field, and (3) herbicide tolerance serves as a marker gene for various studies, e.g. in risk assessments. Other field-tested traits are quality improvement (ca. 20 % of all trials), and marker gene (ca. 10%).
The comparison of public and private activities shows that 61 public institutions account for 29 % and 88 private companies for 71 % of all known field trials. The public sector activity is higher in North America (28 % of the notifiers) than in EU/EFTA (17 %). In the Pacific Rim, the public sector is responsible for 79 % of the trials, while in Central and Southern America 83 % of the trials are carried out by the private sector (mainly counter season evaluation as mentioned above). Table 4.1: Field trials with genetically modified plants according to the crop and the geopolitical area
* others includes: birch (7), chicory (5), lettuce (5), petunia (5), cucumber (4), apple (3), cauliflower (3), sunflower (3), walnut (3), carrot (2), chrysanthemum (2), gerbera (2), sugarcane (2), asparagus (1), brassica sp. (1), cabbage (1), eggplant (1), eucalyptus (1), kiwi (1), papaya (1), peanut (1), plum tree (1), spruce (1) and strawberry (1). Adapted from [20]. Table 4.2: Field trials with genetically modified selected crops according to tested trait.
Adapted from [20]. Disease and pest resistance in bold. 4.4 CommercializationThe release of transgenic crops is an important test for the efficacy of the engineered traits under field conditions. If these tests are successful and enough experience has been gained, an applicant may apply for a commercialization permit. A permit is granted if the results of field trials and laboratory tests have shown that production and consumption of the transgenic crop does not pose risks for the consumers or for the environment. In the USA, the Animal and Plant Health Inspection Service (APHIS) must confirm that the transgenic crop does not pose a plant pest risk and is therefore not subject to regulation. In addition, the new variety must be cleared by the Environmental Protection Agency (EPA) if the plant has pesticidal traits, or by the Food and Drug Administration (FDA). [47] In the European Union (EU), commercialization is regulated under the directive 90/220/EC. If a permit for market introduction is granted in one member state of the EU, the new variety can be commercialized in all member states, but every member may comment on the planned introduction. If one member state objects and no agreement is reached, the European Commission or finally the Council of Ministers decides on the commercialization. [50] Several transgenic crop varieties have obtained a commercialization permit. These include tomato, potato, maize, cotton, squash, soybeans, flax, oilseed rape and tobacco varieties. The altered traits are extended shelf-life, herbicide tolerance, insect resistance, virus resistance, altered processing traits and modified oil profile (see table 4.4 for details) Table 4.4. Transgenic crops with commercialization permit granted.
Sources: For USA [51], for Canada [52] and Canadian Biotech News on Internet, for EU [53] As shown in table 4.4, the great majority of transgenic varieties have been commercialized in the USA. In the European Union (EU), only one variety has obtained a permit for commercial sales (herbicide tolerant tobacco), but apparently this product has not been placed on the market. An additional permit for herbicide tolerant swede rape in the EU is expected. All commercialized insect-resistant crops have been engineered with Bacillus thuringiensis (Bt) δ-endotoxin genes. The potato variety is resistant to Colorado potato beetle, cotton to the cotton bollworm, tobacco budworm, and pink bollworm, and maize to the European corn borer and other moth pests. The European corn borer alone is estimated to cause some US$ 1 billion in crop losses annually to U.S. farmers. The market introduction of these crops has been supported by EPA officials because of the opportunity to reduce chemical-insecticide risks. [54] The transgenic virus resistant squash variety has been transformed with viral coat proteins from the two squash pathogens watermelon mosaic virus 2 and zucchini yellow mosaic virus. The new variety now resists infection by these two viruses. [55] It is still to early to tell whether the transgenic varieties now present on the market will be an economic success. The first genetically engineered food on the market, Calgenes FlavrSavr tomatoes, trademarked as MacGregor tomatoes and labeled as "grown from genetically engineered seeds", have reached the first groceries during summer 1994. As tomatoes soften and rot very quickly, they are usually picked when still green and artificially ripened using ethylene gas before selling. The Calgene tomatoes can ripe naturally because they soften more slowly and are then transported to the stores. MacGregor's tomatoes were found to be slightly better than supermarket tomatoes by the U.S. magazine Consumer Report, but not enough to justify their premium price. [56] It is very likely that the number of commercialized transgenic crops will rise steadily during the next years. In the field of pest and disease resistance, protection against various insect pests by expression of Bt genes and coat protein-mediated protection against viruses in different crop species will continue to be the most often engineered traits in the beginning. Within some years, probably also varieties with enhanced resistance against fungi (e.g. mediated by chitinases and other pathogenesis related proteins from plants) and bacteria (e.g. conferred by cecropins or antimicrobial proteins from plants) may become available. Other applications of transgenic plants expected to reach the marketplace within a few years include cotton producing altered fibers, crops with improved nutritional value, and plants producing cheap vaccines, pharmaceuticals or biodegradable plastic. [57]
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|




 Produkten.
Produkten.